Clinically-supported embrace® dressings prevent and reduce lipohypertrophy and fibrosis at insulin pump and insertion sites

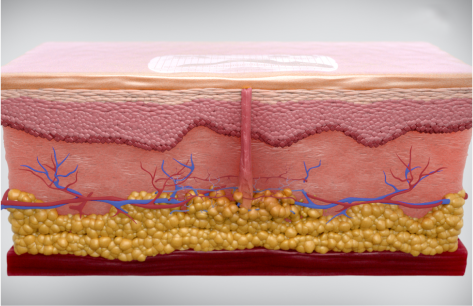
Two leading skin complications for people with diabetes are scarring and lipohypertrophy (LH), caused by insulin injections. The repeated trauma from insulin injections and pump insertions cause inflammation and immune responses, major contributing factors to both scarring and LH. The accumulation of scarring and LH form tissue bundles that have a known negative effect on insulin efficacy, absorption, and blood glucose levels.
Compression of the wound environment with embrace® has been proven to significantly reduce scar formation, therefore mitigating dermal and subcutaneous damage at the injection or insertion site for insulin-users.
Prevalence of scar tissue & LH

64%
of all insulin-users suffer from LH, making it the leading complication from injecting insulin.
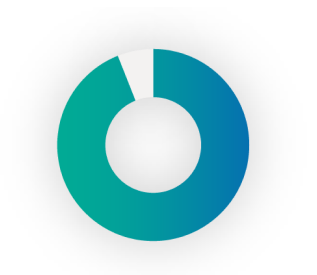
94%
of T1 pump users develop scarring from insertions.
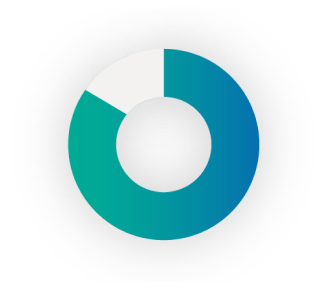
86%
of people with T1 diabetes have scarring and/or LH.
Impact on diabetes control
Patients with tissue bundles consumed 36% more insulin than patients without.

0.55%
higher A1C levels in patients with tissue bundles

6x
higher risk of unexplained hypoglycemia in patients with tissue bundles
49.1%
of patients with LH had glycemic variability (GV), 7x higher than those without
In a 2022 study, Stanford Medicine concluded that using embrace® prevented inflammation, fibrosis, and adipogenesis from injections. In addition, embrace® was able to significantly reduce existing inflammation and fibrosis. Results were confirmed using a variety of techniques including histology, mechanical testing, and advanced cellular signaling analysis. These results support the use of embrace to prevent and heal existing scar tissue and LH.
Preventing or healing existing scar tissue and LH can improve insulin absorption, leading to improved glycemic control, lower A1C levels, and better health outcomes

Benefits of embrace®
For prevention:
embrace® creates compression at and below the surface of the injection and insertion sites, which has been clinically-proven to reduce the inflammation and immune responses responsible for formation of excessive scar tissue and LH. Compression that extends below the surface of the skin prevents fibrotic scarring by reducing expression of TGF-β1, preserving skin health. Furthermore, compression addresses the major mechanisms of LH by reducing immune cell mediated inflammation and adipogenesis. For insulin-users, embrace® aims to preserve the healthy tissue necessary for consistent insulin absorption.
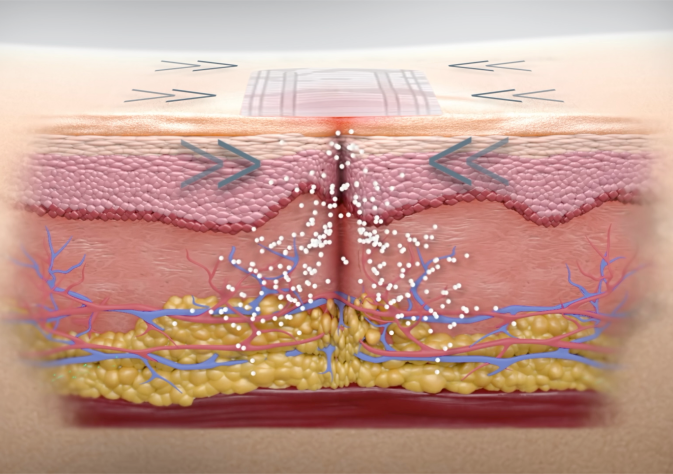
For recovery:
embrace® provides stabilization at the injection and insertion sites, healing existing scar tissue and LH allowing healthy tissue to form in its place. The reduction of immune cell mediated inflammation and adipogenesis, as provided by compression, even reduces the size of fat cells that do form.
FDA-cleared

embrace is the only FDA-cleared device clinically proven to prevent and heal scar tissue and LH, available over-the-counter.

Neodyne surveyed 273 T1 diabetes patients, inclusive of injectors and pumpers. Participants were educated on the embrace® technology, then surveyed about their interest in the diabetes application of embrace®.
The objective of this study was to validate consumer interest, purchase intent, and usage assumptions to validate market opportunity assessment. Additionally, the study sought to understand the existing landscape of consumer pain points, education, and habits. Finally the study looked to understand differences among patient behaviors, adoption, motivation, and compliance.
Key findings
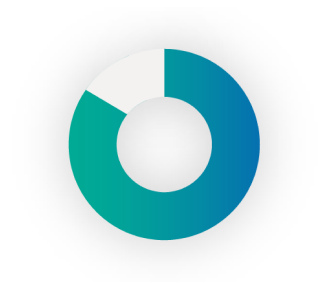
86%
of respondents have some amount of scarring or LH

92%
of respondents have problems with or cannot use at least one insulin delivery area
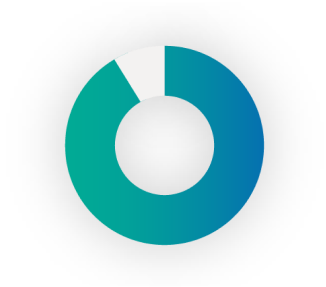
88%
of patients said they would wear embrace® for Prevention for 9 out of 12 months at $20/month
Comfortable to wear
In a randomized, controlled clinical trial of over 375 applications, embrace® was rated as comfortable to wear in 95% of applications, encouraging high patient adherence key to optimal usage.
Seamless treatment integration
Clinical rotation guidelines are the foundation of daily diabetes care. embrace® treatment adheres to global clinical rotation guidelines.
Long-lasting 10 day wear
embrace® is quick and easy to apply. Once applied, each dressing lasts approximately 10 days and is then discarded. The patient rotates and repeats at the next injection site to prevent and heal scar tissue and LH.
Recommend embrace® to your patients
embrace® is supported by leading physicians and certified diabetes educators (CDEs). Refer to the evidence page for clinical studies.



